Kirsty O'Connor/PA Images via Getty Images
- The head of the UK’s vaccine taskforce said Tuesday that a “mix and match” trial will combine both Pfizer and AstraZeneca’s COVID-19 vaccines.
- The trial will test whether combining the two shots works better than administering them individually.
- AstraZeneca and Oxford’s vaccine has not yet been approved by UK regulators, but once it is, the trials will kick off.
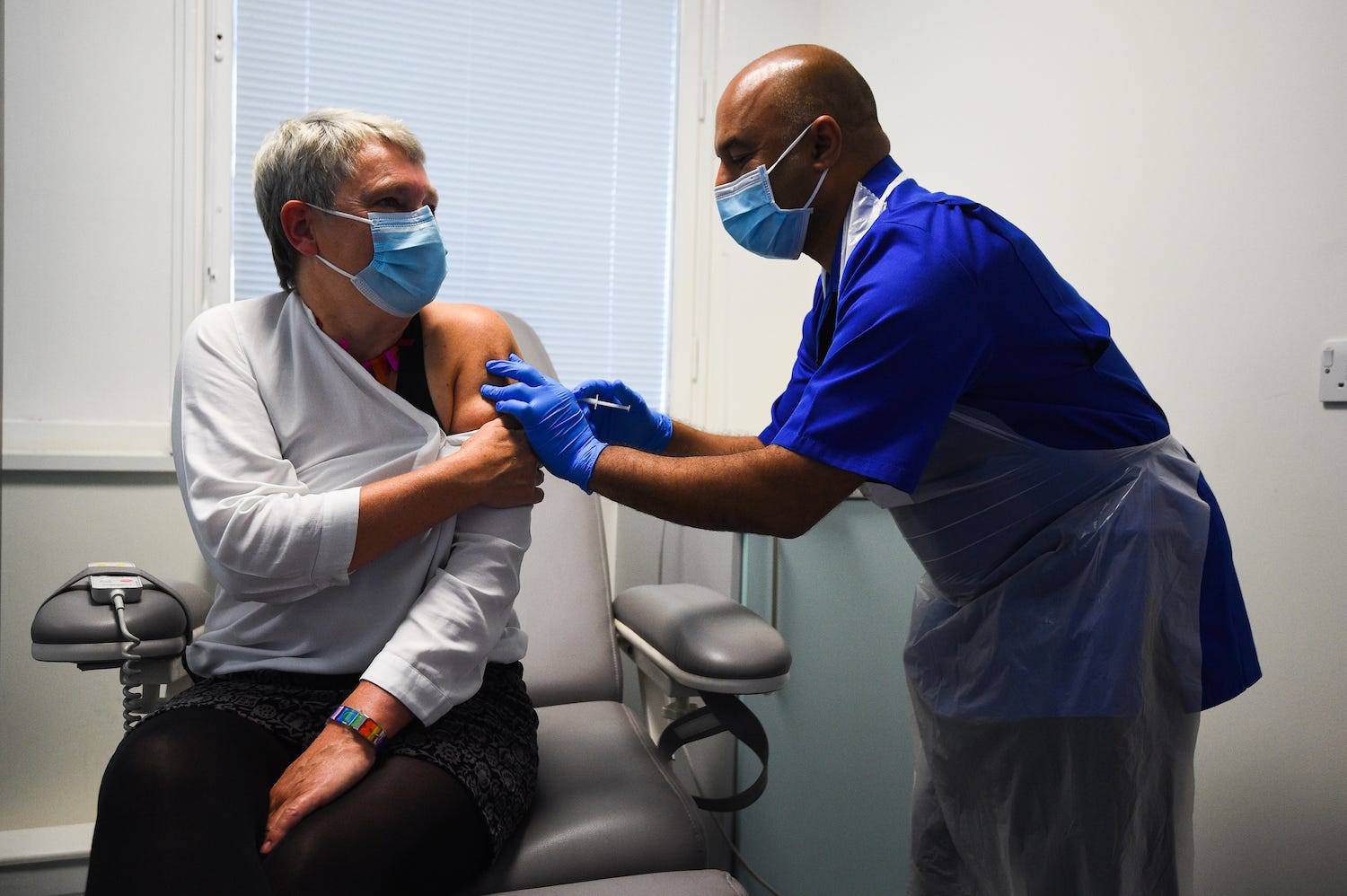
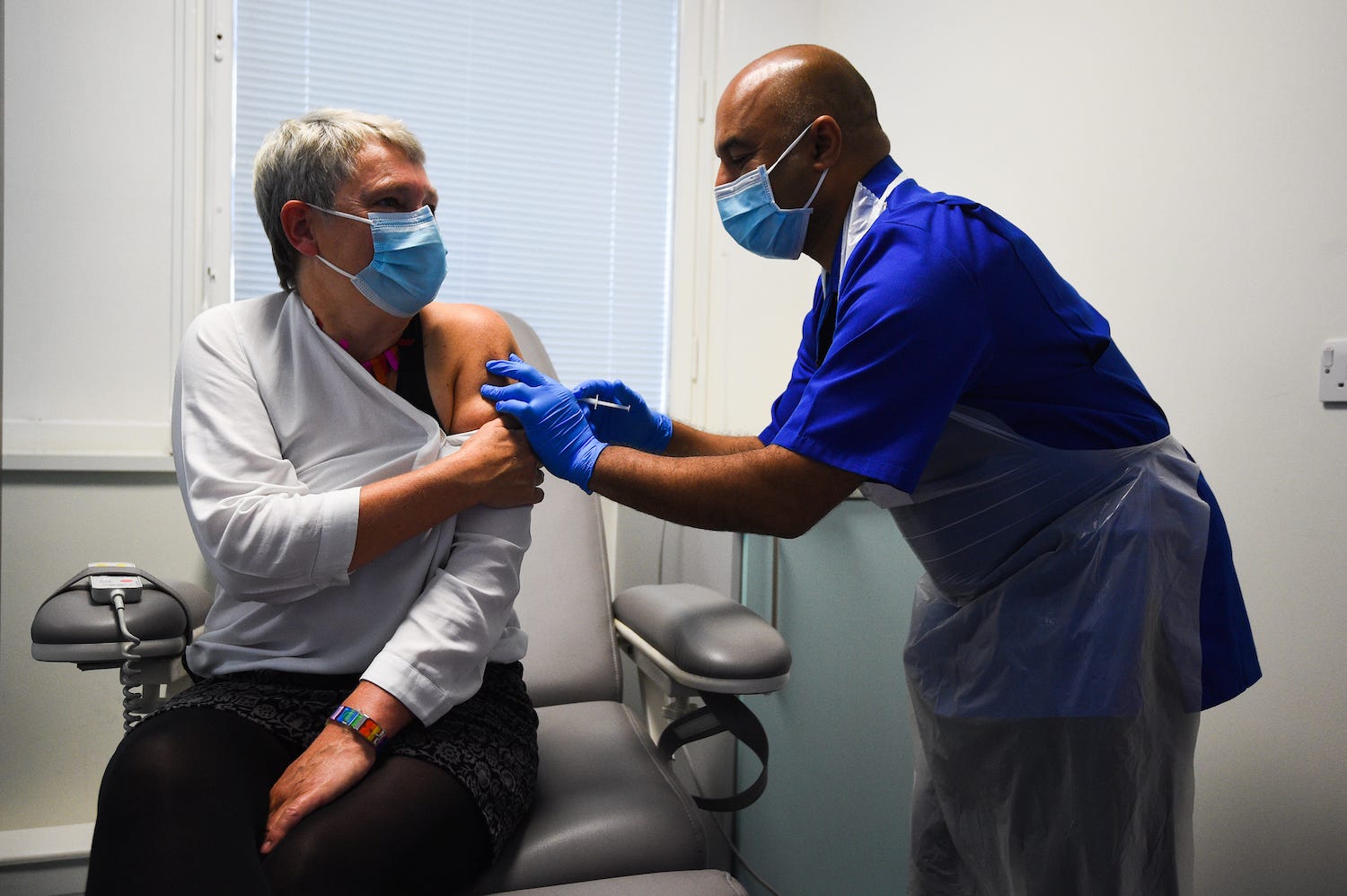
- Pfizer’s COVID-19 vaccine has been approved in the UK, and the first shots were given out Tuesday.
- During the trials, patients will receive one dose of the Pfizer shot and another of the vaccine made by AstraZeneca and Oxford University. The two shots trigger different immune responses.
- Visit Business Insider’s homepage for more stories.
The UK is planning to combine the Pfizer and AstraZeneca vaccines in trials next year to see if both shots together produce a stronger immune response than giving them individually.
Patients who participate in January’s “mix and match” trial – which will begin when AstraZeneca and Oxford’s vaccine is approved, will receive one dose of the Pfizer shot and another of the Oxford shot.
Pfizer’s vaccine has been approved in the UK, and the first shots were given out Tuesday. The US is awaiting approval of the shot, and Americans could be vaccinated as early as Friday.
If the vaccine developed by US biotech firm Moderna gets approval from UK regulators, this will also be included in the trial, Kate Bingham, the head of the British government’s vaccine taskforce, said Tuesday.
Both Pfizer’s and Moderna’s vaccine were 95% effective in late-stage trial results. Oxford and AstraZeneca said their vaccine was 62% effective if patients get two full doses, but 90% effective when they get a half-strength version of the first dose.
Kate Bingham, chair of the UK's vaccine taskforce, said: "The idea is that you can maximize the strength of that immune response to protect people.
"It means mix and matching vaccines," she said at the launch of a report on the group's progress.
"So you do a prime with one vaccine and then the second - whether it's 28 days or two months or whatever the agreed periods would be - would be with a different vaccine."
Both vaccines work in different ways. Typically, mRNA vaccines, such as Pfizer's, produce a greater antibody response, whereas viral-based vaccines such as AstraZeneca's give a bigger cellular response.
"Antibodies block the uptake of viruses into cells and the cellular T-cells identify those cells that have been infected and take them out. You ideally want to have both," Bingham said.
The UK government has ordered 40 million doses of Pfizer's vaccine and 100 million of AstraZeneca's shot.
Clive Dix, deputy chair of the taskforce, said at the event: "No one's ever done it live and since we'll have safe vaccines available we should do that study, because then we have the ability to actually produce better immune responses."
Coronavirus has infected more than 1.7 million people in the UK and killed more 61,000, according to COVID-19 statistics on the UK government website.